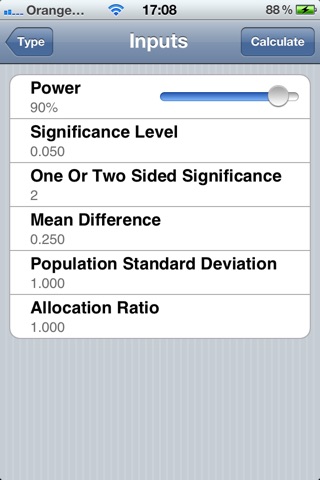
SampSize is an application to assist in the design of clinical trials by calculating the sample size using inputs provided by the user.
Features calculations for:
- Normal and binary endpoints
- Parallel and crossover designs
- Superiority, Non-Inferiority, Equivalence, Bio-equivalence and Precision objectives
SampSize calculates either the required sample size for a desired power, or calculates the power of a trial for a given sample size. It allows users to specify inputs such as significance level, power, mean difference (or limit), standard deviation, and the allocation ratio.
Developed in collaboration with Dr Steven A Julious, Medical Statistics Group, School of Health and Related Research, The University of Sheffield.
References:
- Julious, SA. Sample sizes for clinical trails. Chapman and Hall, 2009.
- Julious, S. J. (2004). Tutorial in Biostatistics: Sample sizes for clinical trials with Normal Data. Statistics in Medicine, 23, 1921-86.
- Julious, S. A. & Campbell, M. J. (2012). Tutorial in biostatistics: sample sizes for parallel group clinical trials with binary data. Statistics in Medicine, doi:10.1002/sim.5381.
- Julious SA & Owen R. Sample size calculations for non-inferiority studies with binary outcomes. Statistical Methods in Medical Research (DOI: 10.1177/0962280210378945).
- Julious SA. An introduction to statistics in early phase trials. Wiley, 2010.

